Thomson's discovery of the electron completely changed the way people viewed atoms. Up until the end of the 19th century, atoms were thought to be tiny solid spheres. In 1903, Thomson proposed a model of the atom consisting of positive and negative charges, present in equal amounts so that an atom would be electrically neutral. Electron Fiddle lets you create and play with small Electron experiments. It greets you with a quick-start template after opening – change a few things, choose the version of Electron you want to run it with, and play around. Then, save your Fiddle either as a GitHub Gist or to a local folder. Once pushed to GitHub, anyone can quickly try. 1 day ago In many textbooks, energy changes of the hydrogen atom are attributed to the electron transitioning between energy levels. However, the energy itself is that of the whole system (proton+electron) s. Atom Under The Microscope Electron & Atomic Force Microscopy What is an Atom? Essentially, an atom is the smallest unit of an element that retains the properties of the same element (iron, copper, carbon etc). This means that divided further, its components (electrons, protons, and neutrons) do not retain the properties of the element.
Understanding the Atom
The nucleus of an atom is surround by electrons that occupy shells, or orbitals of varying energy levels.
The ground state of an electron, the energy level it normallyoccupies, is the state of lowest energy for that electron.

There is also a maximum energy that each electron canhave and still be part of its atom. Beyond that energy, the electronis no longer bound to the nucleus of the atom and it is considered tobe ionized.
When an electron temporarily occupies an energy state greater thanits ground state, it is in an excited state. An electron canbecome excited if it is given extra energy, such as if it absorbs a photon, or packet, of light, or collides with a nearby atom or particle.
Each orbital has a specific energy associated with it. For anelectron to be boosted to an orbital with a higher energy, it mustovercome the difference in energy between the orbital it is in, and theorbital to which is is going. This means that it must absorb a photonthat contains precisely that amount of energy, or take exactly thatamount of energy from another particle in a collision.
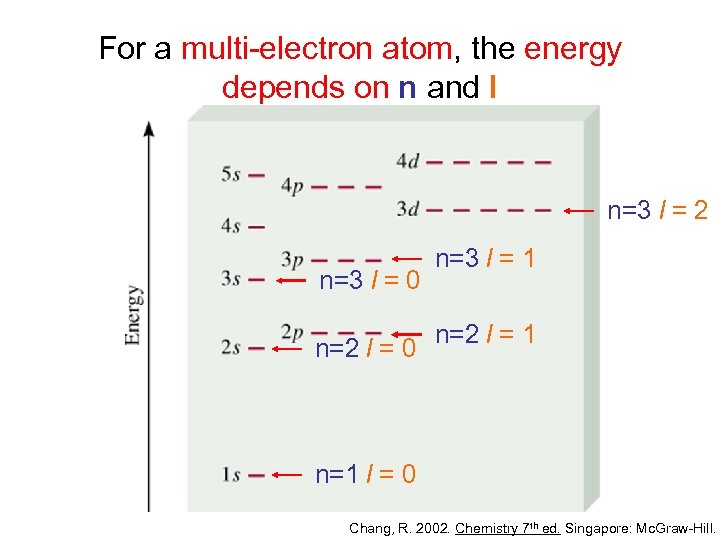
Electrons do not stay in excited states for very long - they soonreturn to their ground states, emitting a photon with the same energy asthe one that was absorbed.
Transitions among the various orbitals are unique for each elementbecause the energy levels are uniquely determined by the protons andneutrons in the nucleus. When the electrons of a certain atom returnto lower orbitals from excited states, the photons they emit haveenergies that are characteristic of that kind of atom. This gives eachelement a unique fingerprint, making it possible to identify theelements present in a container of gas, or even a star.
Updated: November 2013
Atom is a hackable text editor for the 21st century, built on Electron, and based on everything we love about our favorite editors. We designed it to be deeply customizable, but still approachable using the default configuration.
Visit atom.io to learn more or visit the Atom forum.
Follow @AtomEditor on Twitter for importantannouncements.
This project adheres to the Contributor Covenant code of conduct.By participating, you are expected to uphold this code. Please report unacceptable behavior to atom@github.com.
Documentation
If you want to read about using Atom or developing packages in Atom, the Atom Flight Manual is free and available online. You can find the source to the manual in atom/flight-manual.atom.io.
The API reference for developing packages is also documented on Atom.io.
Installing
Prerequisites
macOS
Download the latest Atom release.
Atom will automatically update when a new release is available.
Windows
Download the latest Atom installer. AtomSetup.exe is 32-bit. For 64-bit systems, download AtomSetup-x64.exe.
Atom will automatically update when a new release is available.
You can also download atom-windows.zip (32-bit) or atom-x64-windows.zip (64-bit) from the releases page.The .zip version will not automatically update.

Using Chocolatey? Run cinst Atom to install the latest version of Atom.
Linux
Atom is only available for 64-bit Linux systems.
Configure your distribution's package manager to install and update Atom by following the Linux installation instructions in the Flight Manual. You will also find instructions on how to install Atom's official Linux packages without using a package repository, though you will not get automatic updates after installing Atom this way.
Archive extraction
An archive is available for people who don't want to install atom as root.
Atom Electrons Protons
This version enables you to install multiple Atom versions in parallel. It has been built on Ubuntu 64-bit,but should be compatible with other Linux distributions.
- Install dependencies (on Ubuntu):
sudo apt install git gconf2 gconf-service libgtk2.0-0 libudev1 libgcrypt20 libnotify4 libxtst6 libnss3 python3 gvfs-bin xdg-utils libcap2- (If the
python3package isn't available, or is too old (Python 3 should be >= 3.5), eitherpython2orpython(2.6 or 2.7) will work in its place.)
- (If the
- Download
atom-amd64.tar.gzfrom the Atom releases page. - Run
tar xf atom-amd64.tar.gzin the directory where you want to extract the Atom folder. - Launch Atom using the installed
atomcommand from the newly extracted directory.
The Linux version does not currently automatically update so you will need torepeat these steps to upgrade to future releases.
Building
Electron Atom Diagram
Discussion
- Discuss Atom on our forums or on GitHub Discussions
- Chat about Atom on our Slack team -- instructions for joining
Electron Atom Diagram
License
When using the Atom or other GitHub logos, be sure to follow the GitHub logo guidelines.




