A Fluorine atom, for example, requires the following ionization energy to remove the outermost electron. F + IE → F + + e − IE = 17.4228 eV. The ionization energy associated with removal of the first electron is most commonly used. The nth ionization energy refers to the amount of energy required to remove an electron from the species with.
- The second ionization energy of aluminum is larger than the first, and the third ionization energy is even larger. Although it takes a considerable amount of energy to remove three electrons from an aluminum atom to form an Al 3+ ion, the energy needed to break into the filled-shell configuration of the Al 3+ ion is astronomical.
- It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy (with the exception of Helium and Neon).
These tables list values of molar ionization energies, measured in kJ⋅mol−1. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the neutral atoms. The second, third, etc., molar ionization energy applies to the further removal of an electron from a singly, doubly, etc., charged ion. For ionization energies measured in the unit eV, see Ionization energies of the elements (data page). All data from rutherfordium onwards is predicted.
1st–10th ionisation energies[edit]
| number | symbol | name | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | hydrogen | 1312.0 | |||||||||
| 2 | He | helium | 2372.3 | 5250.5 | ||||||||
| 3 | Li | lithium | 520.2 | 7298.1 | 11,815.0 | |||||||
| 4 | Be | beryllium | 899.5 | 1757.1 | 14,848.7 | 21,006.6 | ||||||
| 5 | B | boron | 800.6 | 2427.1 | 3659.7 | 25,025.8 | 32,826.7 | |||||
| 6 | C | carbon | 1086.5 | 2352.6 | 4620.5 | 6222.7 | 37,831 | 47,277.0 | ||||
| 7 | N | nitrogen | 1402.3 | 2856 | 4578.1 | 7475.0 | 9444.9 | 53,266.6 | 64,360 | |||
| 8 | O | oxygen | 1313.9 | 3388.3 | 5300.5 | 7469.2 | 10,989.5 | 13,326.5 | 71,330 | 84,078.0 | ||
| 9 | F | fluorine | 1681.0 | 3374.2 | 6050.4 | 8407.7 | 11,022.7 | 15,164.1 | 17,868 | 92,038.1 | 106,434.3 | |
| 10 | Ne | neon | 2080.7 | 3952.3 | 6122 | 9371 | 12,177 | 15,238 | 19,999.0 | 23,069.5 | 115,379.5 | 131,432 |
| 11 | Na | sodium | 495.8 | 4562 | 6910.3 | 9543 | 13,354 | 16,613 | 20,117 | 25,496 | 28,932 | 141,362 |
| 12 | Mg | magnesium | 737.7 | 1450.7 | 7732.7 | 10,542.5 | 13,630 | 18,020 | 21,711 | 25,661 | 31,653 | 35,458 |
| 13 | Al | aluminium | 577.5 | 1816.7 | 2744.8 | 11,577 | 14,842 | 18,379 | 23,326 | 27,465 | 31,853 | 38,473 |
| 14 | Si | silicon | 786.5 | 1577.1 | 3231.6 | 4355.5 | 16,091 | 19,805 | 23,780 | 29,287 | 33,878 | 38,726 |
| 15 | P | phosphorus | 1011.8 | 1907 | 2914.1 | 4963.6 | 6273.9 | 21,267 | 25,431 | 29,872 | 35,905 | 40,950 |
| 16 | S | sulfur | 999.6 | 2252 | 3357 | 4556 | 7004.3 | 8495.8 | 27,107 | 31,719 | 36,621 | 43,177 |
| 17 | Cl | chlorine | 1251.2 | 2298 | 3822 | 5158.6 | 6542 | 9362 | 11,018 | 33,604 | 38,600 | 43,961 |
| 18 | Ar | argon | 1520.6 | 2665.8 | 3931 | 5771 | 7238 | 8781 | 11,995 | 13,842 | 40,760 | 46,186 |
| 19 | K | potassium | 418.8 | 3052 | 4420 | 5877 | 7975 | 9590 | 11,343 | 14,944 | 16,963.7 | 48,610 |
| 20 | Ca | calcium | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 | 10,496 | 12,270 | 14,206 | 18,191 | 20,385 |
| 21 | Sc | scandium | 633.1 | 1235.0 | 2388.6 | 7090.6 | 8843 | 10,679 | 13,310 | 15,250 | 17,370 | 21,726 |
| 22 | Ti | titanium | 658.8 | 1309.8 | 2652.5 | 4174.6 | 9581 | 11,533 | 13,590 | 16,440 | 18,530 | 20,833 |
| 23 | V | vanadium | 650.9 | 1414 | 2830 | 4507 | 6298.7 | 12,363 | 14,530 | 16,730 | 19,860 | 22,240 |
| 24 | Cr | chromium | 652.9 | 1590.6 | 2987 | 4743 | 6702 | 8744.9 | 15,455 | 17,820 | 20,190 | 23,580 |
| 25 | Mn | manganese | 717.3 | 1509.0 | 3248 | 4940 | 6990 | 9220 | 11,500 | 18,770 | 21,400 | 23,960 |
| 26 | Fe | iron | 762.5 | 1561.9 | 2957 | 5290 | 7240 | 9560 | 12,060 | 14,580 | 22,540 | 25,290 |
| 27 | Co | cobalt | 760.4 | 1648 | 3232 | 4950 | 7670 | 9840 | 12,440 | 15,230 | 17,959 | 26,570 |
| 28 | Ni | nickel | 737.1 | 1753.0 | 3395 | 5300 | 7339 | 10,400 | 12,800 | 15,600 | 18,600 | 21,670 |
| 29 | Cu | copper | 745.5 | 1957.9 | 3555 | 5536 | 7700 | 9900 | 13,400 | 16,000 | 19,200 | 22,400 |
| 30 | Zn | zinc | 906.4 | 1733.3 | 3833 | 5731 | 7970 | 10,400 | 12,900 | 16,800 | 19,600 | 23,000 |
| 31 | Ga | gallium | 578.8 | 1979.3 | 2963 | 6180 | ||||||
| 32 | Ge | germanium | 762 | 1537.5 | 3302.1 | 4411 | 9020 | |||||
| 33 | As | arsenic | 947.0 | 1798 | 2735 | 4837 | 6043 | 12,310 | ||||
| 34 | Se | selenium | 941.0 | 2045 | 2973.7 | 4144 | 6590 | 7880 | 14,990 | |||
| 35 | Br | bromine | 1139.9 | 2103 | 3470 | 4560 | 5760 | 8550 | 9940 | 18,600 | ||
| 36 | Kr | krypton | 1350.8 | 2350.4 | 3565 | 5070 | 6240 | 7570 | 10,710 | 12,138 | 22,274 | 25,880 |
| 37 | Rb | rubidium | 403.0 | 2633 | 3860 | 5080 | 6850 | 8140 | 9570 | 13,120 | 14,500 | 26,740 |
| 38 | Sr | strontium | 549.5 | 1064.2 | 4138 | 5500 | 6910 | 8760 | 10,230 | 11,800 | 15,600 | 17,100 |
| 39 | Y | yttrium | 600 | 1180 | 1980 | 5847 | 7430 | 8970 | 11,190 | 12,450 | 14,110 | 18,400 |
| 40 | Zr | zirconium | 640.1 | 1270 | 2218 | 3313 | 7752 | 9500 | ||||
| 41 | Nb | niobium | 652.1 | 1380 | 2416 | 3700 | 4877 | 9847 | 12,100 | |||
| 42 | Mo | molybdenum | 684.3 | 1560 | 2618 | 4480 | 5257 | 6640.8 | 12,125 | 13,860 | 15,835 | 17,980 |
| 43 | Tc | technetium | 702 | 1470 | 2850 | |||||||
| 44 | Ru | ruthenium | 710.2 | 1620 | 2747 | |||||||
| 45 | Rh | rhodium | 719.7 | 1740 | 2997 | |||||||
| 46 | Pd | palladium | 804.4 | 1870 | 3177 | |||||||
| 47 | Ag | silver | 731.0 | 2070 | 3361 | |||||||
| 48 | Cd | cadmium | 867.8 | 1631.4 | 3616 | |||||||
| 49 | In | indium | 558.3 | 1820.7 | 2704 | 5210 | ||||||
| 50 | Sn | tin | 708.6 | 1411.8 | 2943.0 | 3930.3 | 7456 | |||||
| 51 | Sb | antimony | 834 | 1594.9 | 2440 | 4260 | 5400 | 10,400 | ||||
| 52 | Te | tellurium | 869.3 | 1790 | 2698 | 3610 | 5668 | 6820 | 13,200 | |||
| 53 | I | iodine | 1008.4 | 1845.9 | 3180 | |||||||
| 54 | Xe | xenon | 1170.4 | 2046.4 | 3099.4 | |||||||
| 55 | Cs | caesium | 375.7 | 2234.3 | 3400 | |||||||
| 56 | Ba | barium | 502.9 | 965.2 | 3600 | |||||||
| 57 | La | lanthanum | 538.1 | 1067 | 1850.3 | 4819 | 5940 | |||||
| 58 | Ce | cerium | 534.4 | 1050 | 1949 | 3547 | 6325 | 7490 | ||||
| 59 | Pr | praseodymium | 527 | 1020 | 2086 | 3761 | 5551 | |||||
| 60 | Nd | neodymium | 533.1 | 1040 | 2130 | 3900 | ||||||
| 61 | Pm | promethium | 540 | 1050 | 2150 | 3970 | ||||||
| 62 | Sm | samarium | 544.5 | 1070 | 2260 | 3990 | ||||||
| 63 | Eu | europium | 547.1 | 1085 | 2404 | 4120 | ||||||
| 64 | Gd | gadolinium | 593.4 | 1170 | 1990 | 4250 | ||||||
| 65 | Tb | terbium | 565.8 | 1110 | 2114 | 3839 | ||||||
| 66 | Dy | dysprosium | 573.0 | 1130 | 2200 | 3990 | ||||||
| 67 | Ho | holmium | 581.0 | 1140 | 2204 | 4100 | ||||||
| 68 | Er | erbium | 589.3 | 1150 | 2194 | 4120 | ||||||
| 69 | Tm | thulium | 596.7 | 1160 | 2285 | 4120 | ||||||
| 70 | Yb | ytterbium | 603.4 | 1174.8 | 2417 | 4203 | ||||||
| 71 | Lu | lutetium | 523.5 | 1340 | 2022.3 | 4370 | 6445 | |||||
| 72 | Hf | hafnium | 658.5 | 1440 | 2250 | 3216 | ||||||
| 73 | Ta | tantalum | 761 | 1500 | ||||||||
| 74 | W | tungsten | 770 | 1700 | ||||||||
| 75 | Re | rhenium | 760 | 1260 | 2510 | 3640 | ||||||
| 76 | Os | osmium | 840 | 1600 | ||||||||
| 77 | Ir | iridium | 880 | 1600 | ||||||||
| 78 | Pt | platinum | 870 | 1791 | ||||||||
| 79 | Au | gold | 890.1 | 1980 | ||||||||
| 80 | Hg | mercury | 1007.1 | 1810 | 3300 | |||||||
| 81 | Tl | thallium | 589.4 | 1971 | 2878 | |||||||
| 82 | Pb | lead | 715.6 | 1450.5 | 3081.5 | 4083 | 6640 | |||||
| 83 | Bi | bismuth | 703 | 1610 | 2466 | 4370 | 5400 | 8520 | ||||
| 84 | Po | polonium | 812.1 | |||||||||
| 85 | At | astatine | 899.003 | |||||||||
| 86 | Rn | radon | 1037 | |||||||||
| 87 | Fr | francium | 380 | |||||||||
| 88 | Ra | radium | 509.3 | 979.0 | ||||||||
| 89 | Ac | actinium | 499 | 1170 | 1900 | 4700 | ||||||
| 90 | Th | thorium | 587 | 1110 | 1978 | 2780 | ||||||
| 91 | Pa | protactinium | 568 | 1128 | 1814 | 2991 | ||||||
| 92 | U | uranium | 597.6 | 1420 | 1900 | 3145 | ||||||
| 93 | Np | neptunium | 604.5 | 1128 | 1997 | 3242 | ||||||
| 94 | Pu | plutonium | 584.7 | 1128 | 2084 | 3338 | ||||||
| 95 | Am | americium | 578 | 1158 | 2132 | 3493 | ||||||
| 96 | Cm | curium | 581 | 1196 | 2026 | 3550 | ||||||
| 97 | Bk | berkelium | 601 | 1186 | 2152 | 3434 | ||||||
| 98 | Cf | californium | 608 | 1206 | 2267 | 3599 | ||||||
| 99 | Es | einsteinium | 619 | 1216 | 2334 | 3734 | ||||||
| 100 | Fm | fermium | 627 | 1225 | 2363 | 3792 | ||||||
| 101 | Md | mendelevium | 635 | 1235 | 2470 | 3840 | ||||||
| 102 | No | nobelium | 642 | 1254 | 2643 | 3956 | ||||||
| 103 | Lr | lawrencium | 470 | 1428 | 2228 | 4910 | ||||||
| 104 | Rf | rutherfordium | 580 | 1390 | 2300 | 3080 | ||||||
| 105 | Db | dubnium | 665 | 1547 | 2378 | 3299 | 4305 | |||||
| 106 | Sg | seaborgium | 757 | 1733 | 2484 | 3416 | 4562 | 5716 | ||||
| 107 | Bh | bohrium | 740 | 1690 | 2570 | 3600 | 4730 | 5990 | 7230 | |||
| 108 | Hs | hassium | 730 | 1760 | 2830 | 3640 | 4940 | 6180 | 7540 | 8860 | ||
| 109 | Mt | meitnerium | 800 | 1820 | 2900 | 3900 | 4900 | |||||
| 110 | Ds | darmstadtium | 960 | 1890 | 3030 | 4000 | 5100 | |||||
| 111 | Rg | roentgenium | 1020 | 2070 | 3080 | 4100 | 5300 | |||||
| 112 | Cn | copernicium | 1155 | 2170 | 3160 | 4200 | 5500 | |||||
| 113 | Nh | nihonium | 707.2 | 2309 | 3226 | 4382 | 5638 | |||||
| 114 | Fl | flerovium | 832.2 | 1600 | 3370 | 4400 | 5850 | |||||
| 115 | Mc | moscovium | 538.3 | 1760 | 2650 | 4680 | 5720 | |||||
| 116 | Lv | livermorium | 663.9 | 1330 | 2850 | 3810 | 6080 | |||||
| 117 | Ts | tennessine | 736.9 | 1435.4 | 2161.9 | 4012.9 | 5076.4 | |||||
| 118 | Og | oganesson | 860.1 | 1560 | ||||||||
| 119 | Uue | ununennium | 463.1 | 1700 | ||||||||
| 120 | Ubn | unbinilium | 563.3 | 895– 919 | ||||||||
| 121 | Ubu | unbiunium | 429.4 | 1110 | 1710 | 4270 | ||||||
| 122 | Ubb | unbibium | 545 | 1090 | 1848 | 2520 |
11th–20th ionisation energies[edit]
| number | symbol | name | 11th | 12th | 13th | 14th | 15th | 16th | 17th | 18th | 19th | 20th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | Na | sodium | 159,076 | |||||||||
| 12 | Mg | magnesium | 169,988 | 189,368 | ||||||||
| 13 | Al | aluminium | 42,647 | 201,266 | 222,316 | |||||||
| 14 | Si | silicon | 45,962 | 50,502 | 235,196 | 257,923 | ||||||
| 15 | P | phosphorus | 46,261 | 54,110 | 59,024 | 271,791 | 296,195 | |||||
| 16 | S | sulfur | 48,710 | 54,460 | 62,930 | 68,216 | 311,048 | 337,138 | ||||
| 17 | Cl | chlorine | 51,068 | 57,119 | 63,363 | 72,341 | 78,095 | 352,994 | 380,760 | |||
| 18 | Ar | argon | 52,002 | 59,653 | 66,199 | 72,918 | 82,473 | 88,576 | 397,605 | 427,066 | ||
| 19 | K | potassium | 54,490 | 60,730 | 68,950 | 75,900 | 83,080 | 93,400 | 99,710 | 444,880 | 476,063 | |
| 20 | Ca | calcium | 57,110 | 63,410 | 70,110 | 78,890 | 86,310 | 94,000 | 104,900 | 111,711 | 494,850 | 527,762 |
| 21 | Sc | scandium | 24,102 | 66,320 | 73,010 | 80,160 | 89,490 | 97,400 | 105,600 | 117,000 | 124,270 | 547,530 |
| 22 | Ti | titanium | 25,575 | 28,125 | 76,015 | 83,280 | 90,880 | 100,700 | 109,100 | 117,800 | 129,900 | 137,530 |
| 23 | V | vanadium | 24,670 | 29,730 | 32,446 | 86,450 | 94,170 | 102,300 | 112,700 | 121,600 | 130,700 | 143,400 |
| 24 | Cr | chromium | 26,130 | 28,750 | 34,230 | 37,066 | 97,510 | 105,800 | 114,300 | 125,300 | 134,700 | 144,300 |
| 25 | Mn | manganese | 27,590 | 30,330 | 33,150 | 38,880 | 41,987 | 109,480 | 118,100 | 127,100 | 138,600 | 148,500 |
| 26 | Fe | iron | 28,000 | 31,920 | 34,830 | 37,840 | 44,100 | 47,206 | 122,200 | 131,000 | 140,500 | 152,600 |
| 27 | Co | cobalt | 29,400 | 32,400 | 36,600 | 39,700 | 42,800 | 49,396 | 52,737 | 134,810 | 145,170 | 154,700 |
| 28 | Ni | nickel | 30,970 | 34,000 | 37,100 | 41,500 | 44,800 | 48,100 | 55,101 | 58,570 | 148,700 | 159,000 |
| 29 | Cu | copper | 25,600 | 35,600 | 38,700 | 42,000 | 46,700 | 50,200 | 53,700 | 61,100 | 64,702 | 163,700 |
| 30 | Zn | zinc | 26,400 | 29,990 | 40,490 | 43,800 | 47,300 | 52,300 | 55,900 | 59,700 | 67,300 | 171,200 |
| 36 | Kr | krypton | 29,700 | 33,800 | 37,700 | 43,100 | 47,500 | 52,200 | 57,100 | 61,800 | 75,800 | 80,400 |
| 38 | Sr | strontium | 31,270 | |||||||||
| 39 | Y | yttrium | 19,900 | 36,090 | ||||||||
| 42 | Mo | molybdenum | 20,190 | 22,219 | 26,930 | 29,196 | 52,490 | 55,000 | 61,400 | 67,700 | 74,000 | 80,400 |
21st–30th ionisation energies[edit]
| number | symbol | name | 21st | 22nd | 23rd | 24th | 25th | 26th | 27th | 28th | 29th | 30th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | Sc | scandium | 582,163 | |||||||||
| 22 | Ti | titanium | 602,930 | 639,294 | ||||||||
| 23 | V | vanadium | 151,440 | 661,050 | 699,144 | |||||||
| 24 | Cr | chromium | 157,700 | 166,090 | 721,870 | 761,733 | ||||||
| 25 | Mn | manganese | 158,600 | 172,500 | 181,380 | 785,450 | 827,067 | |||||
| 26 | Fe | iron | 163,000 | 173,600 | 188,100 | 195,200 | 851,800 | 895,161 | ||||
| 27 | Co | cobalt | 167,400 | 178,100 | 189,300 | 204,500 | 214,100 | 920,870 | 966,023 | |||
| 28 | Ni | nickel | 169,400 | 182,700 | 194,000 | 205,600 | 221,400 | 231,490 | 992,718 | 1,039,668 | ||
| 29 | Cu | copper | 174,100 | 184,900 | 198,800 | 210,500 | 222,700 | 239,100 | 249,660 | 1,067,358 | 1,116,105 | |
| 30 | Zn | zinc | 179,100 | |||||||||
| 36 | Kr | krypton | 85,300 | 90,400 | 96,300 | 101,400 | 111,100 | 116,290 | 282,500 | 296,200 | 311,400 | 326,200 |
| 42 | Mo | molybdenum | 87,000 | 93,400 | 98,420 | 104,400 | 121,900 | 127,700 | 133,800 | 139,800 | 148,100 | 154,500 |
References[edit]
- Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). 'Transactinides and the future elements'. In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN1-4020-3555-1. (for predictions)
- Cotton, Simon (2006). Lanthanide and Actinide Chemistry. John Wiley & Sons Ltd.
- Fricke, Burkhard (1975). 'Superheavy elements: a prediction of their chemical and physical properties'. Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013. (for predictions)
1) Which of the following order is correct for the first ionization energies of following elements? (EAMCET 2009)
1) B < Be < N < O
2) Be < B < N < O
3) B < Be < O < N
4) B < O < Be < N
Logic:
* Ionization energy, in general, increases with decrease in the atomic radius across the period from left to right. However there are exceptions.
* s-orbitals have greater penetration power than p-orbitals. Hence the removal of electrons from s-orbitals require more energy.
* The electrons in half filled orbitals are stable as they experience less repulsion. If there is another electron sharing same orbital, then they will be destabilized due to repulsion from each other.
* Above atoms belong to same period (2nd) of periodic table.
| Group number | 2 | 13 | 15 | 16 |
| Element | 4Be | 5B | 7N | 8O |
| electronic configuration | 2s2 | 2s22p1 | 2s22p3 | 2s22p4 |
Solution:
* Boron, B is smaller than beryllium, Be atom. Hence we expect increase in ionization energy from Be to B.
However, Be atom has greater ionization energy than B atom. The reason is - in case of Beryllium, the last electron is in the s-orbital and in Boron, the last electron is in the p-orbital. We know that removal of electron from s-orbital requires more energy than the electron from p-orbital.
* 2s22p3 configuration is more stable than 2s22p4 due to half filled p-sublevel. The electrons in the p-orbital in N atom experience less repulsion, thus more stable and more ionization energy. Whereas in case of O atom the 4th p-electron atom can be removed more easily as it experiences more repulsion from the electrons in the p-orbitals already present. Hence nitrogen, Oxygen atom has less ionization energy than Nitrogen atom.
Conclusion:
The correct ionization energy order is: B < Be < O < N
(new) Click here to see 3d Interactive Solved Question paper
Followup Practice questions on Ionization potential
2) The first ionization potential of four consecutive elements, present in the second period of the periodic table are 8.3, 11.3, 14.5 and 13.6 eV respectively. Which one of the following is the first ionization potential (in eV) of nitrogen?
(Eamcet - 2004-M)
1) 13.6
2) 11.3
3) 8.3
4) 14.5
Logic & solution:
The ionization energies increase regularly for the first three elements. Then there is decrease in the IE value from 3rd to 4th element. This indicates, 3rd element must possess stable configuration. Hence the third element is nitrogen.
Conclusion:
The first ionization potential (in eV) of nitrogen is 14.5. The correct option is '4'.
3) The electron configuration of elements A, B and C are [He]2s1, [Ne]3s1 and [Ar]4s1 respectively. Which one of the following order is correct for the first ionization potentials (in kJ mol–1) of A, B and C?
(Eamcet - 2001-E)
1) A > B > C
2) C > B > A
3) B > C >A
4) C > A > C
Logic & solution:
The electronic configurations clearly indicate that they belong to same group of periodic table i.e. 1st group. The atomic size increases with increase in the value of principal quantum number, n of valence orbital (from 2 to 3 to 4). Hence the ionization energy decreases from A to B to C.
Conclusion:
The correct option is '1'.
4) The correct order of second ionization energies of C, N, O and F is:
(IIT JEE 1991)
1) C > N > O > F
2) O > N > F > C
3) O > F > N > C
4) F > O > N > C
Logic & solution:
The second ionization energy refers to the energy required to remove the electron from the corresponding mono-valent cation of the respective atom. The atoms: C, N, O and F belong to 2nd period of the periodic table.
Just like second ionization energy like the first IE is affected by size, effective nuclear charge, type of orbital from which the electron is being removed and electronic configuration.
It is expected to increase from left to right in the periodic table with decrease in the atomic size. However oxygen has greater second ionization energy than fluorine and also nitrogen.
Reason: Since Oxygen atom gets stable electronic configuration, 2s22p3 after removing one electron, the O+ shows greater ionization energy than F+ as well as N+.
| Group number | 14 | 15 | 16 | 17 |
| Element | C | N | O | F |
| electronic configuration | 2s22p2 | 2s22p3 | 2s22p4 | 2s22p5 |
| After removing one electron | | | | ∇ | | | | ∇ | | | | ∇ | | | | ∇ |
| Monovalent cation | C+ | N+ | O+ | F+ |
| electronic configuration | 2s22p1 | 2s22p2 | 2s22p3 | 2s22p4 |
| 2nd IE (kJ mol-1) | 2352 | 2855 | 3388 | 3375 |
Hence the order of second ionization energies of above elements is: O > F > N > C.
Conclusion:
The correct option is '3'.
5)The incorrect statement among the following is:
(IIT JEE 1997)
1) The first ionization potential of Al is less than the first ionization potential of Mg.
2) The second ionization potential of Mg is greater than the second ionization potential Na.
3) The first ionization potential of Na is less than the first ionization potential of Mg.
4) The third ionization potential of Mg is greater than that of Al.
Logic & solution:
The outer electronic configurations of atoms, univalent and divalent cations of elements mentioned in above statement are tabulated below.
| Group number | 1 | 2 | 13 |
| Element | Na | Mg | Al |
| electronic configuration | 2s22p63s1 | 2s22p63s2 | 2s22p63s23p1 |
| After removing one electron | | | | ∇ | | | | ∇ | | | | ∇ |
| univalent cation | Na+ | Mg+ | Al+ |
| electronic configuration | 2s22p6 | 2s22p63s1 | 2s22p63s2 |
| After removing second electron | | | | ∇ | | | | ∇ | | | | ∇ |
| Divalent cation | Na2+ | Mg2+ | Al2+ |
| electronic configuration | 2s22p5 | 2s22p6 | 2s22p63s1 |
Statement-1 is correct since, the electron has to be removed from full filled s-orbital, the first ionization energy of Mg is greater than that of Al.
Statement-2 is incorrect since Na+ has now stable octet configuration(2s22p6) and requires greater energy to remove second electron than in case of Mg+.

Statement-3 is correct because Mg is smaller than Na and has greater effective nuclear charge. Hence the first ionization energy of Mg is higher.
Statement-4 is also correct. It is because of stable octet configuration of Mg2+ formed after removing two electrons from Mg.
Conclusion:
The correct option is 2 ( the statement given is incorrect).
6) The first ionization potentials of Na, Mg, Al and Si are in the order:
(IIT JEE 1988)
1) Na < Mg > Al < Si
2) Na < Mg < Al > Si
3) Na > Mg > Al > Si

4) Na > Mg > Al < Si
Logic & solution:
They belong to same period. We can expect increase in ionization energy from Na to Si. Yet, Mg has greater ionization energy than Al due to 2s22p63s2 configuration. It is more difficult to remove electron from 3s orbital than from 3p orbital since s-orbitals have greater penetration power. Moreover, Mg has stable electronic configuration with full filled 3s orbital.
Conclusion:
The correct first ionization energy order is shown in the option '1'.
7) The first ionization energy in electron volts of nitrogen and oxygen atom's are respectively given by:
(IIT JEE 1987)
a) 14.6, 13.6
b) 13.6, 14.6
c) 13.6, 13.6
d) 14.6, 14.6
Logic & solution:
Since nitrogen has greater ionization energy than oxygen, the correct option is 'a'.
8) Which of the following species has the highest ionization potential?
(EAMCET 1998-E)
1) Li+
2) Mg+
3) Al+
4) Ne
Logic & solution:
Li+ has 1s2 configuration, which is the configuration of He atom. Hence it should possess highest IP value.

Conclusion:
The correct option is '1'.
9) The set representing correct order of first ionization potential is:
(IIT JEE 2001)
1) K > Na > Li
2) Be > Mg > Ca
3) B > C > N
4) Ge > Si > C
Logic & solution:
1) K > Na > Li : Incorrect, since ionization energy decreases down the group with increase in size. These elements belong to same group (1st). The correct order of first ionization energy should be: K < Na < Li.
| Li | | | | ionization energy decreases down the group | | ∇ |
| Na | |
| K |
2) Be > Mg > Ca : Correct. These are 2nd group elements and the order correctly reflects trend in ionization energy.
| Be | | | | ionization energy decreases down the group | | ∇ |
| Mg | |
| Ca |
3) B > C > N: Incorrect. They belong to same period and the order should be reverse since the IP values increase from left to right in a period.
| B | C | N |
| ----------> ionization energy increases | ||
4) Ge > Si > C : Incorrect: Belong to same group (14th).The order must be in reverse.
| C | | | | ionization energy decreases down the group | | ∇ |
| Si | |
| Ge |
Conclusion:
The correct option is '2'.
10) Amongst the following elements (whose electronic configurations are given below), the one having the highest ionization energy is:
(IIT JEE 1990)
a) [Ne] 3s2 3p1
b) [Ar] 3d10 4s2 4p2
c) [Ne] 3s2 3p2
d) [Ne] 3s2 3p3
Logic & solution:
Based on electronic configuration of elements, their positions in the periodic table can be assigned as shown below.
| 13 | 14 | 15 | |
| Period 3 | [Ne] 3s2 3p1 | [Ne] 3s2 3p2 | [Ne] 3s2 3p3 |
| Period 4 | [Ar] 3d10 4s2 4p2 |
The element with electronic configuration [Ne] 3s2 3p3 is on the extreme right and with stable 3p3 configuration (half filled sub level). Hence it should have highest IE value.
Conclusion:
The correct option is 'd'.
11) The increasing order of the first ionization enthalpies of the elements B, P, S and F (lowest first) is:
(AIEEE 2006)
a) F < S < P < B
b) P < S < B < F
c) B < P < S < F
d) B < S < P < F
Logic & solution:
| 13 | 15 | 16 | 17 |
| Period 3 | B (800.6 kJ mol-1) | F (1681 kJ mol-1) | |
| Period 4 | P (1012 kJ mol-1) | S (999.6 kJ mol-1) |
Fluorine, being the smallest has the highest ionization enthalpy among the given elements.
Phosphorus has 3s23p3 configuration with half filled p-sub level. Hence it has higher IP value than Sulfur.
Ionization Energy Of Fluorine Ion
Conclusion:
The correct option is 'd'.
12) The correct order of ionization energy for the following species is:
(AdiChemistry IIT JEE NEET 2018)
1) He < Li+ < H-
2) H- < Li+ < He
3) H < Li+ < He
4) H- < He < Li+
Logic & solution:
All the species have the same electronic configuration i.e. 1s2. But theenergy required to remove the outer electron increases with decrease in the negative charge (or increase in the positive charge).
Conclusion:
The correct option is 4.
13) Which of the following atoms has the highest first ionization energy?
(IIT JEE MAIN 2016)
(1) Na
(2) K
(3) Sc
(4) Rb
Logic & solution:
Ionization Energy Of Fluorine
All the atoms, except scandium belong to alkali metals. Scandium belongs to 3rd group (II B). it is a of d-block element - a transition metal. Its atomic radius is smaller than the other given atoms. Hence it is expected to have higher ionization energy among the given options.
| 1 (I A) | 3 (II B) | |
| Period 3 | Na (11) ---> [Ne] 3s1 | |
| Period 4 | K (19) ---> [Ar] 4s1 | Sc (21) ---> [Ar] 3d1 5s2 |
| Period 5 | Rb (37) ---> [Kr] 5s1 |
Conclusion:
The correct option is 3.
IONIZATION ENERGY - FOLLOWUP PRACTICE QUESTIONS & PROBLEMS WITH ANSWERS
1) The increasing order of second ionization energies of Na, Ne, Mg and Al is __________ .
Answer: Na > Ne > Al > Mg
Information: The second ionization energies (in kJ mol-1) are: Na (4563), Ne (3963), Al (1820), Mg (1450).
2) The correct increasing order of effective nuclear charge among the elements O, F, Ne, C, N is _______ .
Answer: C < N < O < F < Ne
Explanation: On moving from left to right in the periodic table in a period, the effective nuclear charge increases.
3) Lowest IP will be shown by the element having the configuration:
A) [He] 2s2
B) [He] 2s2 2p2
C) 1s2
D) [He] 2s2 2p3
Answer: B
4) Which element in the periodic table has the highest ionization potential?
Answer: Helium (He) has the highest first ionization energy (IE1).
5) The electronic configuration with the highest ionization enthalpy is ________
Answer: 1s2.
6) Which of the following has maximum ionisation potential ?
A) F
B) Mg2+
C) He+
D) Li+
Answer: D
Information: Ionization potential values in kJ/mol: F (1680), Mg2+ (1450), He+ (5250), Li+ (7292)
7) Which of the following is the correct sequence for increasing order (i.e. smallest to largest) of first ionization energy (IE1)?
A) O > C > F
B) O > N > F
C) N > C > F
D) F > N > O
Answer: D
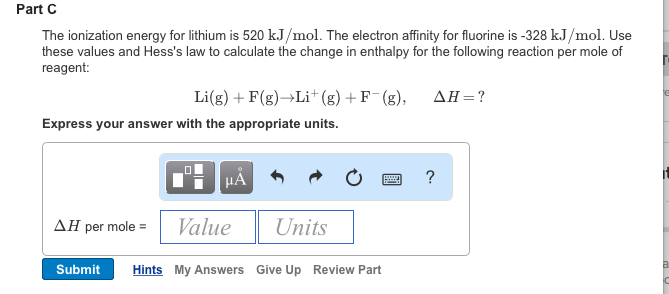
2) What is the relation between ionization potential and effective nuclear charge experienced by an electron in nth orbit?
3) What is the general trend observed in ionization energy in alkali and alkaline earth metals?




