Naturally occurring iodine has an atomic mass of 126.9045. A 12.3849 g sample of iodine is accidentally contaminated with 1.00070 g of 129 I, a synthetic radioisotope of iodine used in the.
- Consider the following data for iodine: atomic mass 126.90 € mol 2.66 electronegativity electron affinity 295.2 mol lonization energy 1008.4 heat of fusion 7.76 You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy?
- Atomic Mass of Iodine. Atomic mass of Iodine is 126.90447 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams.
- Iodine (I) - Iodine with the symbol 'I' is a dark gray or purple blackish nonmetallic element with atomic number 53 in the Periodic Table. Learn iodine atomic number, atomic mass of iodine and iodine uses.
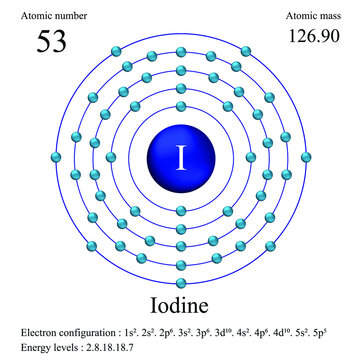
We elaborate the uses of Iodine and atomic properties with characteristics. Iodine is a chemical element with a violet appearance (Gas) Violet gray (Solid) with atomic number 53. Its symbol is I and it belongs to the group of halogens and its usual state in nature is solid. Iodine is located at position 53 on the periodic table.
You Can Visit Our Managed: Periodic Table Main Page

On this page you can discover the chemical properties of iodine and information about iodine and other elements of the periodic table such as bromine, astate, tellurium or xenon. You will also learn what iodine is for and you will know what its uses through its properties associated with iodine such as its atomic number or the usual state in which iodine can be found.
You will be able to see qualities of iodine such as its melting and boiling point, its magnetic properties or what its chemical symbol is. Also, here you will find information about its atomic properties such as the distribution of electrons in iodine atoms and other properties.
Iodine Atomic Mass Number
For some elements, part of this information is unknown. In these cases we show the properties attributed to them.
Properties of iodine
Iodine Atomic Mass Number
Elements of the group of halogens such as iodine occur as chemically active diatomic molecules. The name halogen comes from the Greek and its meaning is “salt former”. They are halogen elements, including iodine, they are oxidants. Many organic synthetic compounds and some natural organic compounds contain halogens such as iodine. These types of compounds are known as halogenated compounds.
The state of iodine in its natural form is solid. Iodine is a chemical element with a violet appearance (Gas) Purplish gray (Solid) and belongs to the group of halogens. The atomic number of iodine is 53. The chemical symbol for iodine is I. The melting point of iodine is 355.95 degrees Kelvin or 83.8 degrees Celsius, or degrees Centigrade. The boiling point of iodine is 457.4 degrees Kelvin or 185.25 degrees Celsius or degrees Centigrade.
Iodine is a mineral that our body needs for its proper functioning and can be found in food. Through the following link, you can find a list of foods with iodine .
Atomic Properties of Iodine
The atomic mass of an element is determined by the total mass of neutrons and protons that can be found in a single atom belonging to this element. Regarding the position where to find iodine within the periodic table of elements, iodine is found in group 17 and period 5. Iodine has an atomic mass of 126.90447 u.
The electron configuration of iodine is [Kr] 4d105s25p5. The electronic configuration of the elements determines the way in which the electrons are structured in the atoms of an element. The mean radius of iodine is 140 pm, its atomic radius or Bohr radius is 115 pm, its covalent radius is 133 pm, and its Van der Waals radius is 198 pm. Iodine has a total of 53 electrons whose distribution is as follows: In the first shell it has 2 electrons, in the second it has 8 electrons, in its third shell it has 18 electrons, in the fourth, 18 electrons and in the fifth shell it has 7 electrons.
You Can Visit Our Managed: Periodic Table Main Page
Characteristics of iodine
Below you can see a table that shows the main characteristics of iodine.
| Iodine | ||
|---|---|---|
| Chemical symbol | I | |
| Atomic number | 53 | |
| Group | 17 | |
| Period | 5 | |
| Appearance | Violet (Gas) Violet gray (Solid) | |
| Block | p | |
| Density | 4,940 kg / m3 | |
| Atomic mass | 126.90447 u | |
| Medium radius | 140 pm | |
| Atomic radio | 115 | |
| Covalent radius | 133 pm | |
| Van der Waals radio | 198 pm | |
| Electronic configuration | [Kr] 4d105s25p5 | |
| Electrons per shell | 2, 8, 18, 18, 7 | |
| Oxidation states | -1, 1, 3, 5, 7 | |
| Oxide | strong acid | |
| Crystal structure | orthorhombic | |
| State | solid | |
| Melting point | 355.95 K | |
| Boiling point | 457.4 K | |
| Heat of fusion | 7,824 kJ / mol | |
| Electronegativity | 2.66 | |
| Specific heat | 145 J / (Kkg) | |
| Electric conductivity | 8.0 × 10-8S / m | |
| Thermal conductivity | 0.449 W / (Km) | |
You Can Visit Our Managed: Periodic Table Main Page
Atomic Mass and Nuclear Binding Energy for I-125 (Iodine)
Abstract

This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope I-125 (Iodine, atomic number Z = 53, mass number A = 125).

- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter




